Abstract
Background. Despite a large body of evidence that hydroxyurea is effective for sickle cell anemia (SCA), utilization with the "maximum tolerated dose" regimen has been low.1 Hydroxyurea therapy for SCA increases hemoglobin F, reduces pain crises, acute chest syndrome and blood transfusions, and possibly increases survival.2 The increase in hemoglobin F with hydroxyurea is related to the plasma level achieved, which depends on the dose.3 Hydroxyurea is typically initiated at 15 mg/kg/day followed by dose escalations up to 35 mg/kg/day if tolerated - the "maximum tolerated dose" approach used in the seminal Multicenter Study of Hydroxyurea (MSH) in the 1990's.4 With this approach, neutropenia and thrombocytopenia are limitations to achieving maximal dose and frequent blood count monitoring is required.These considerations limit the use of hydroxyurea in areas of the world where frequent blood monitoring is not feasible, and they also cause primary care providers in the US to hesitate to prescribe the medication.
Methods. We recently reported a trial of hydroxyurea (500 mg/day orally) in 48 adults with SCA in Ibadan, Nigeria - a "fixed low-dose" approach.5 In the present report, we compare hematologic responses in 38 per protocol patients from the Ibadan study (received at least five of the planned six 4-weekly supplies of hydroxyurea)5 to the responses in 152 patients in the MSH study.6
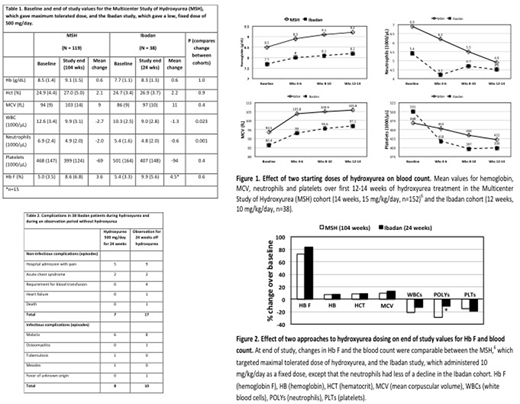
Results. Baseline hematologic values for the two studies are shown in Table 1. Over the first 12 to 14 weeks of hydroxyurea, responses in hemoglobin, mean corpuscular volume (MCV), and platelets were similar in the two studies (Figure 1). At end-of-study (24 weeks Ibadan and 104 weeks MSH) responses in hemoglobin F and complete blood count were similar, except that the decline in neutrophil count in the Ibadan cohort was significantly less than the decline in MSH after the Bonferroni correction for multiple comparisons (Table 1and Figure 2). At this time the median (range) dose of hydroxyurea per body weight was 10 (7-14) mg/kg per day in the 38 Ibadan patients versus 20 (2.5-35) mg/kg per day in the 119 patients who remained on hydroxyurea in the MSH study.6 We performed a review of the medical records in the Ibadan patients. There were seven acute non-infectious complications in the 38 patients while taking fixed low-dose hydroxyurea for 24 weeks (admission for pain or acute chest syndrome) versus 17 while being observed off hydroxyurea for 24 weeks (pain, acute chest syndrome, need for blood transfusion, heart failure or death) (Table 2). Incidence of infections was similar during 24 weeks of fixed low-dose hydroxyurea and during 24 weeks of observation off hydroxyurea (Table 2).
Discussion. These findings suggest that a "fixed low-dose" regimen may have substantial clinical benefit. Such a regimen would have a low chance of inducing cytopenias, would be straightforward for primary care providers to prescribe and would be ideal to test in combination therapy regimens for SCA. It would also be cost-efficient and affordable, especially for patients in low and middle-income countries where access to health insurance is lacking. We propose that the clinical efficacy of "fixed low-dose" hydroxyurea for SCA should be investigated further. We believe that a randomized comparison of the efficacy and safety of the "fixed low-dose" and "maximum tolerated dose" regimens should be a high priority.
References
Stettler N, McKiernan CM, Melin CQ, Adejoro OO, Walczak NB. Proportion of adults with sickle cell anemia and pain crises receiving hydroxyurea. JAMA 2015;313:1671-2.
Platt OS. Hydroxyurea for the treatment of sickle cell anemia. N Engl J Med 2008;358:1362-9.
Charache S, Dover GJ, Moore RD, et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood 1992;79:2555-65.
Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 1995;332:1317-22.
Tayo BO, Akingbola TS, Saraf SL, et al. Fixed Low-Dose Hydroxyurea for the Treatment of Adults with Sickle Cell Anemia in Nigeria. Am J Hematol 2018.
Charache S, Barton FB, Moore RD, et al. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive "switching" agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine (Baltimore) 1996;75:300-26.
Hsu:Global Blood Therapeutics: Research Funding; Astra Zeneca: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Ironwood: Research Funding; Emmi: Consultancy; Hilton Publishing: Consultancy; Gerson Lehman Group: Consultancy; Guidepoint: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal